DUZEY ULTRACOT “Skinny" Neurosurgical Patties
ISO9001:2015 Requirement
Click on the green button below to provide feedback on our products and services.
The good, the not so good and the bad!
Medical Device Regulation Requirement
For any reportable incidences with these medical devices click on the red button below to notify ourselves and the manufacture DUZEY Medical.
Product Overview:
Patty Front & Back
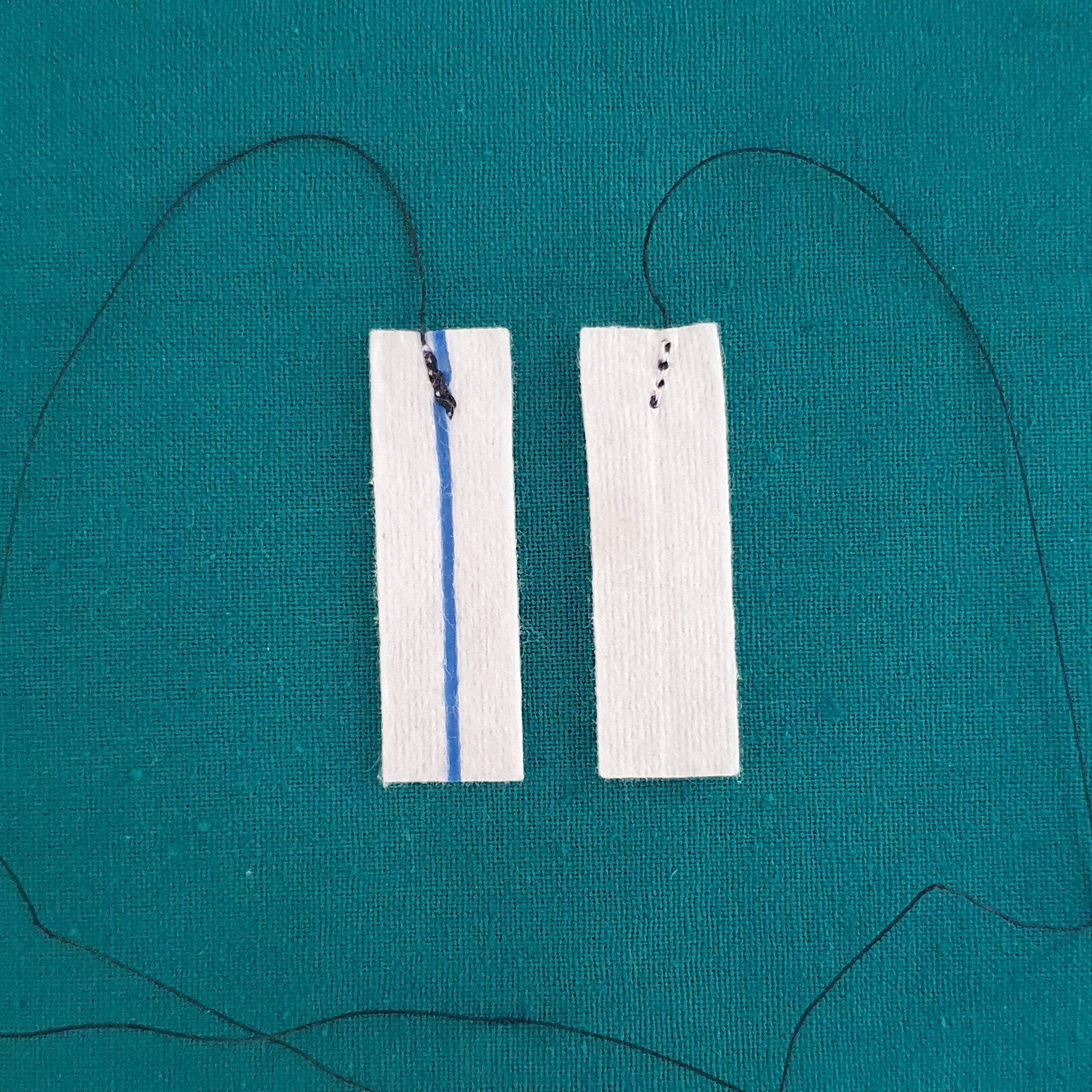
Each Patty has:
- X-Ray detectable strip on one side.
- 30cm long black medical grade synthetic polyester yarn for identification purposes only and not for removing the pattie from the wound.
- Yarn knott securely attaching it to the patty
Patty Sterile Pouches
10 sterile patties are mounted on the counting card, which is supplied inside a paper envelope which in tern is inside an easy peel sterile pouch i.e. each sterile pouch contains 10 patties.
DUZEY ULTRACOT Skinny Neurosurgical Patties are available in the following shape & sizes:
Square Patties:
DSK55, 5 x 5 mm (1/4" x 1/4") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK1212, 12 x12 mm (1/2" x 1/2") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK2020, 20 x 20 mm (3/4" x 3/4") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK2525, 25 x 25 mm (1" x 1") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK7575, 70 x 70 mm (2 3/4" x 2 3/4") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 10 pouches per box.
Rectangular Patties:
DSK1225, 12 x 25 mm (1/2" x 1") DUZEY ULTRACOT Skinny Patties 10units/sterile pouch, 20 pouches per box.
DSK1240, 12 x40 mm (1/2" x 1 1/2") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK1250, 12 x 50 mm (1/2" x 2") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK1275, 12 x 75 mm (1/2" x 3") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 20 pouches per box.
DSK2575, 25 x 75 mm (1" x 3") DUZEY ULTRACOT Skinny Patties 10 units/sterile pouch, 10 pouches per box.
DSK740, 7 x 40 mm (1/4" x 1 1/2") DUZEY ULTRACOT Skinny Patties 10units/sterile pouch, 20 pouches per box.
DSK775, 7 x 75 mm (1/4" x 3") DUZEY ULTRACOT Skinny Patties 10units/sterile pouch, 20 pouches per box.
How to purchase
These products can be purchased direct by clicking here and also, with authorised access, from the following online NHS catalogues:
- Edge4Health online Catalogue.
Product Training & Education Zone
The Product Training and Education Zone on our website provides resouces for organisations who are either customers of Anser Medical Ltd or have agreed to evaluate our product range prior to instigating switching to our brand.
The resource includes product presentations and training videos, technical documentation and evaluation forms.
This is a password protected area, for access
contact Anser Medical Ltd.










